Shale oil extraction
| Shale oil extraction | |
|---|---|
 Shell's experimental in situ oil shale facility, Piceance Basin, Colorado, United States |
|
| Process type | Chemical |
| Industrial sector(s) | Chemical industry, oil industry |
| Main technologies or sub-processes | Kiviter, Galoter, Petrosix, Fushun, Shell ICP |
| Feedstock | Oil shale |
| Product(s) | Shale oil |
| Leading companies | Royal Dutch Shell, Eesti Energia, Viru Keemia Grupp, Petrobras, Fushun Mining Group |
| Main facilities | Fushun Shale Oil Plant, Narva Oil Plant, Petrosix, Stuart Shale Oil Plant |
Shale oil extraction is an industrial process for unconventional oil production. This process converts kerogen in oil shale into shale oil by pyrolysis, hydrogenation, or thermal dissolution. The resultant shale oil is used as fuel oil or upgraded to meet refinery feedstock specifications by adding hydrogen and removing sulfur and nitrogen impurities.
Shale oil extraction is usually performed above ground (ex situ processing) by mining the oil shale and then treating it in processing facilities. Other modern technologies perform the processing underground (on-site or in situ processing) by applying heat and extracting the oil via oil wells.
Though the earliest description of the process dates back to the 10th century AD, the first patent for the process was granted in 1684. Extraction industries and innovations became widespread during the 19th century. Though the process lost ground in the mid-20th century following the discovery of large reserves of conventional oil, the beginning of 21st Century, has seen it regain attention as a potential substitute for crude oil due to petroleum prices. As of 2010, shale oil extraction is being undertaken in Estonia, Brazil, and China. Its economic viability varies with the ratio of local energy input costs to energy output value. National energy security issues have also played a role in its development. Critics of shale oil extraction pose questions about environmental management issues, such as waste disposal, extensive water use and waste water management, and air pollution.
Contents |
History
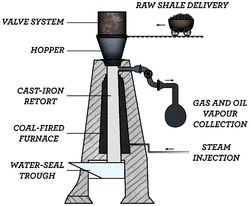
A number of shale oil extraction technologies have evolved over a period of time.[1][2] In the 10th century, a method of extracting oil from "some kind of bituminous shale" was described by the Arabian physician Masawaih al-Mardini (Mesue the Younger).[3] The first shale oil extraction patent was granted by the British Crown in 1694 to three people who had "found a way to extract and make great quantities of pitch, tarr, and oyle out of a sort of stone".[1][4][5] Modern industrial extraction of shale oil originated in France with the implementation of a process invented by Alexander Selligue in 1838 and about a decade later in Scotland by implementation of the process invented by James Young.[1][6] During the late 19th century, shale oil extraction plants were built in Australia, Brazil, Canada, and the United States.[7] The 1894 invention of the Pumpherston retort (also known as the Bryson retort) marked the separation of oil shale industry from the coal industry.[1]
China (Manchuria), Estonia, New Zealand, South Africa, Spain, Sweden, and Switzerland began extracting shale oil in the early 20th century. However, crude oil discoveries in Texas during the 1920s and in the Middle East during mid-century brought most oil shale industries to a halt.[7][8][9][10] In 1944, the United States restarted shale oil extraction as part of its Synthetic Liquid Fuels Program. These industries continued until oil prices fell sharply in the 1980s.[8][11][12] The last oil shale retort in the United States, operated by Unocal Corporation, closed in 1991.[11][12] The United States' oil-shale development program was restarted in 2003, followed by a commercial leasing program in 2005 permitting the extraction of oil shale and oil sands on federal lands in accordance with the Energy Policy Act of 2005.[13]
As of 2010[update], shale oil extraction is in operation in Estonia, Brazil, and China.[14][15][16] While, Australia, United States and Canada have tested shale oil extraction techniques with demonstration projects and are planning implementation on a commercial basis, Morocco and Jordan are also planning to start shale oil production.[7][11][16][17][18] Only four technologies are in commercial use; namely Kiviter, Galoter, Fushun, and Petrosix.[2][15]
Process principle
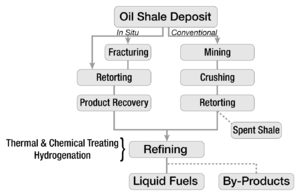
Shale oil extraction process decomposes oil shale and converts kerogen in oil shale into shale oil—a petroleum-like synthetic crude oil. The process is conducted by pyrolysis, hydrogenation, or thermal dissolution.[19][20] The most common extraction method is pyrolysis (also known as retorting). In this process, oil shale is heated until its kerogen decomposes into vapors of a condensable shale oil and non-condensable combustible oil shale gas. Oil vapors and oil shale gas are collected and cooled, causing the shale oil to condense.[19] In addition, oil shale processing produces spent shale, which is a solid residue. Spent shale may contain char (some authors use the terms coke residue or semi-coke instead of char) —a carbonaceous residue formed from kerogen. Depending on the exact composition of oil shale, other useful by-products are also generated during this process. These include ammonia, sulfur, aromatic compounds, pitch, asphalt, and waxes.[12]
Pyrolysis is an endothermic process that requires an external source of energy. Most technologies use other fossil fuels such as natural gas, oil, or coal to generate heat, but various experimental methods have used electricity, radio frequency, microwaves, or reactive fluids for this purpose.[21] By-products of the retorting process such as oil shale gas and char may be burned as an additional source of energy and the heat contained in spent oil shale and oil shale ash may be reused to pre-heat the raw oil shale.[19]
The temperature at which perceptible decomposition of oil shale occurs depends on the time-scale of the process. In ex situ retorting processes, it begins at 300 °C (570 °F) and proceeds more rapidly and completely at higher temperatures. The rate of decomposition is the highest when the temperature ranges between 480 and 520 °C (900 and 970 °F). The ratio of oil shale gas to shale oil generally increases along with retorting temperatures.[19] For a modern in situ process, which might take several months of heating, decomposition may be conducted at temperatures as low as 250 °C (480 °F). Temperatures below 600 °C (1,110 °F) are preferable, preventing the decomposition of lime stone and dolomite in the rock and thereby limiting carbon dioxide emissions and energy consumption.[22]
Hydrogenation and thermal dissolution (reactive fluid processes) extract the oil using hydrogen donors, solvents, or a combination of these. Thermal dissolution involves the application of solvents at elevated temperatures and pressures, increasing oil output by cracking the dissolved organic matter. Different methods produce shale oil with different properties.[20][23][24][25] The efficiency of extraction processes is often evaluated by comparing their yield to the results of a Fischer Assay performed on a sample of the shale.
Classifications
Industry analysts have created several classifications of the methods by which hydrocarbons are extracted from oil shale.
By process principles: Based on the treatment of raw oil shale by heat and solvents the methods are classified as pyrolysis, hydrogenation, or thermal dissolution.[20]
By location: A frequently used distinction considers whether processing is done above or below ground, and classifies the technologies broadly as ex situ (displaced) or in situ (in place). In ex situ processing, also known as above–ground retorting, the oil shale is mined either underground or at the surface and then transported to a processing facility. In contrast, in situ processing converts the kerogen while it is still in the form of an oil shale deposit, following which it is then extracted via oil wells, where it rises in the same way as conventional crude oil.[21]
By heating method: The heating methods used to decompose oil shale may be classified as direct or indirect. While methods that burn materials or insert heat carriers within the retort are classified as direct, methods that conduct heat through retort walls are described as indirect.[15][21] As of 2009, most of the commercial retorts in operation or under development are direct heating retorts.[15] Another classification is based upon whether the heat is delivered by solids (hot recycled solids methods) or gases. In principle, it is less expensive to deliver heat using solids, especially those already heated by the shale's pyrolysis, as is the case when spent shale particles are used.[21]
By retort style: Based on the materials and methods used to heat the oil shale to an appropriate temperature, its processing technologies have been classified into internal combustion, hot recycled solids, wall conduction, externally–generated hot gas, reactive fluid, and volumetric heating methods.[10][21][26][27] There are many possible realizations and combinations of these methods, which are summarized in the table shown below. Some processing technologies are difficult to classify due to their unique methods of heat input (e.g. ExxonMobil Electrofrac) or due to limited information.[21]
| Classification of processing technologies by heating method and location (according to Alan Burnham)[10][21][26][27] | ||
|---|---|---|
| Heating Method | Above ground (ex situ) | Underground (in situ) |
| Internal combustion | Gas combustion, NTU, Kiviter, Fushun, Union A, Paraho Direct, Superior Direct | Occidental Petroleum MIS, LLNL RISE, Geokinetics Horizontal, Rio Blanco |
| Hot recycled solids (inert or burned shale) |
Alberta Taciuk, Galoter, Lurgi-Ruhrgas, TOSCO II, Chevron STB, LLNL HRS, Shell Spher, KENTORT II | - |
| Conduction through a wall (various fuels) |
Pumpherston, Hom Tov, Fischer Assay, Oil-Tech, EcoShale In-Capsule Process, Combustion Resources | Shell ICP (primary method), American Shale Oil CCR, IEP Geothermic Fuel Cell Process |
| Externally generated hot gas | PetroSIX, Union B, Paraho Indirect, Superior Indirect, Syntec process (Smith process) | Chevron CRUSH, Omnishale, MWE IGE |
| Reactive fluids | IGT Hytort (high-pressure H2), donor solvent processes, Chattanooga fluidized bed reactor | Shell ICP (some embodiments) |
| Volumetric heating | - | radiofrequency, microwave and electric current processes |
By raw oil shale particles' size: The various ex situ processing technologies may be differentiated by the size of the oil shale particles that are fed into the retorts. As a rule, oil shale "lumps" varying in diameter from 10 to 100 millimeters (0.4 to 3.9 in) are used in internal hot gas carrier technologies, while oil shale that has been crushed into particulates less than 10 millimeters (0.4 in) in diameter are used in internal hot solid carrier technologies.[15]
By complexity of technology: In situ technologies are usually classified either as true in situ processes or modified in situ processes. True in situ processes do not involve mining or crushing the oil shale. Modified in situ processes involve drilling and fracturing the target oil shale deposit to create voids for the improved flow of gases and fluids through the deposit, thereby increasing the volume and quality of the shale oil produced.[12]
Ex situ technologies
Internal combustion
Internal combustion technologies burn materials (typically char and oil shale gas) within a vertical shaft retort to supply heat for pyrolysis.[10][21] Typically raw oil shale is fed into the top of the retort and is heated by the rising hot gases, which pass through the descending oil shale, thereby causing decomposition. Shale oil vapors and evolving gases are then moved to a condensing system. Condensed shale oil is collected, while non-condensable gas is recycled and used to carry heat. In the lower part of the retort, spent oil shale is heated to about 900 °C (1,650 °F) to burn off the char. Recycled gas enters the bottom of the retort and cools the spent oil shale.[10][19][28] The Union and Superior multimineral processes depart from this pattern. In the Union process, oil shale is fed through the bottom of the retort and a pump moves it upward.[10] In the Superior multimineral process, oil shale is processed in a horizontal segmented doughnut-shaped traveling-grate retort.[10][22][29]
These processes are thermally efficient, since much of the carbon within the shale is burnt, and can achieve 80-90% of Fischer assay yield.[27] Two well-established shale oil industries use internal combustion technologies: Kiviter process facilities have been operated continuously in Estonia since the 1920s, and a number of Chinese companies operate Fushun process facilities. Their product streams, however, are diluted by combustion exhaust.[27]
Hot recycled solids
Hot recycled solids technologies deliver heat to the shale via solid particles—typically oil shale ash. These technologies usually employ rotating kiln retorts, fed by fine oil shale particles generally having a diameter of less than 10 millimeters (0.4 in); some technologies use particles even smaller than 2.5 millimeters (0.10 in). The particles are heated in a separate chamber or vessel, advantageously preventing the dilution of oil shale gas with combustion exhaust.[10][21]
In the Galoter process, the spent oil shale is burnt in a separate furnace and the resulting hot ash is mixed with oil shale particles to cause decomposition.[30] This process and its modified version, Enefit, have been used in Estonia's Narva Oil Plant for several decades. The TOSCO II process uses hot shale ash and ceramic balls heated by contact with the ash.[12] The distinguishing feature of the Alberta Taciuk process (ATP) is that the entire process occurs in a single rotating multi–chamber horizontal vessel.[12][15] An ATP plant extracted 1.5 million barrels (238.48094×103 m3) of shale oil between 2000 and 2005 at the Stuart Oil Shale Plant, but is now being dismantled.[31]
Conduction through a wall
These technologies transfer heat to the oil shale by conducting it through the retort wall. The shale feed usually consists of fine particles. Their advantage lies in the fact that retort vapors are not combined with combustion exhaust.[10][21] The Combustion Resources process uses a hydrogen–fired rotating kiln, where hot gas is circulated through an outer annulus.[32][33] The Oil-Tech staged electrically heated retort consists of individual inter-connected heating chambers, stacked atop each other.[11][29] Its principal advantage lies in its modular design, which enhances its portability and adaptability.[29] The Red Leaf Resources EcoShale In-Capsule Process combines surface mining with a lower-temperature heating method similar to in situ processes by operating within an earthen impoundment structure. Inside the impoundment, a hot gas circulated by parallel pipes heats the oil shale rubble.[11][34][35] As the impoundment could be constructed in the empty space created by mining, it allows rapid reclamation of the topography.[35]
Externally generated hot gas
In general, externally generated hot gas technologies are similar to internal combustion technologies in that they also process oil shale lumps in vertical shaft kilns. Significantly, though, the heat in these technologies is delivered by gases heated outside the retort vessel, and therefore the retort vapors are not diluted with combustion exhaust.[10][21] The Petrosix process, used at the world's largest operational surface oil shale pyrolysis retort in São Mateus do Sul, Paraná, Brazil, employs this technology.[12][36]
Reactive fluids
Reactive fluid technologies are suitable for processing oil shales with a low hydrogen content. In these technologies, hydrogen gas (H2) or hydrogen donors (chemicals that donate hydrogen during chemical reactions) react with coke precursors (chemical structures in the oil shale that are prone to form char during retorting but have not yet done so). The reaction roughly doubles the yield of oil, depending on the characteristics of oil shale and process technology.[37]
Reactive fluids technologies include the IGT Hytort (high-pressure H2) process, donor solvent processes, and the Chattanooga fluidized bed reactor.[11][21] In the IGT Hytort oil shale is processed in a high-pressure hydrogen environment.[38] The Chattanooga process uses a fluidized bed reactor and an associated hydrogen-fired heater for oil shale thermal cracking and hydrogenation.[11]
In situ technologies
In situ technologies heat oil shale underground by injecting hot fluids into the rock formation, or by using linear or planar heating sources followed by thermal conduction and convection to distribute heat through the target area. Shale oil is then recovered through vertical wells drilled into the formation.[11] These technologies are potentially able to extract more shale oil from a given area of land than conventional ex situ processing technologies, as the wells can reach greater depths than surface mines. They present an opportunity to recover shale oil from low-grade deposits that traditional mining techniques could not extract.[39]
During World War II a modified in situ extraction process was implemented without significant success in Germany.[10] One of the earliest successful in situ processes was the underground gasification by electrical energy (Ljungström method)—a process exploited between 1940 and 1966 for shale oil extraction at Kvarntorp in Sweden.[10][40] Prior to the 1980s, many variations of the in situ process were explored in the United States. The first modified in situ oil shale experiment in the United States was conducted by Occidental Petroleum in 1972 at Logan Wash, Colorado.[12] The newest technologies explore a variety of heat sources and heat delivery systems.
Wall conduction
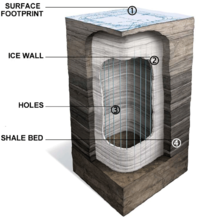
Wall conduction in situ technologies use heating elements or heating pipes placed within the oil shale formation. The Shell in situ conversion process (Shell ICP) uses electrical heating elements for heating the oil shale layer to between 650 and 700 °F (340 and 370 °C) over a period of approximately four years.[41] The processing area is isolated from surrounding groundwater by a freeze wall consisting of wells filled with a circulating super-chilled fluid.[26][42] Disadvantages of this process are large electrical power consumption, extensive water use, and the risk of groundwater pollution.[43] The process, under development since the early 1980s, is tested at the Mahogany test site in the Piceance Basin. 1,700 barrels (270 m3) of oil were extracted in 2004 at a 30-by-40-foot (9.1 by 12 m) testing area.[41][42][44]

In the American Shale Oil CCR Process, superheated steam or another heat transfer medium is circulated through a series of pipes placed below the oil shale layer to be extracted. The system combines horizontal wells, through which steam is passed, and vertical wells, which provide both vertical heat transfer through refluxing of converted shale oil and a means to collect the produced hydrocarbons. Heat is supplied by combustion of natural gas or propane in the initial phase and by oil shale gas at a later stage.[11][45]
The Independent Energy Partners' Geothermic Fuels Cells Process (IEP GFC) extracts shale oil by exploiting a high-temperature stack of fuel cells. The cells, placed in the oil shale formation, are fueled by natural gas during a warm-up period and afterward by oil shale gas generated by its own waste heat.[11][40]
Externally generated hot gas
Externally generated hot gas in situ technologies use hot gases that are heated above-ground and then injected into the oil shale formation. The Chevron CRUSH process, developed by Chevron Corporation in partnership with Los Alamos National Laboratory, injects heated carbon dioxide into the formation via drilled wells and heats the formation through a series of horizontal fractures in which the gas circulates.[46] General Synfuels International has proposed the Omnishale process which involves injecting super-heated air into the oil shale formation.[11][35] Mountain West Energy's In Situ Vapor Extraction process uses similar principles of injection of high-temperature gas.[11][47]
ExxonMobil Electrofrac
ExxonMobil's in situ technology uses electrical heating with elements of both wall conduction and volumetric heating methods. It injects an electrically conductive material such as calcined petroleum coke into the hydraulic fractures created in the oil shale formation which then forms a heating element.[11][48][49] Heating wells are placed in a parallel row with a second horizontal well intersecting them at their toe. This allows opposing electrical charges to be applied at either end.[11][49]
Volumetric heating
The Illinois Institute of Technology developed the concept of oil shale volumetric heating by radio waves (radio frequency processing) at the late 1970s. This technology was further developed by Lawrence Livermore National Laboratory. The oil shale would be heated by vertical electrode arrays. Deeper volumes could be processed at slower heating rates by installations spaced at tens of meters. The concept presumes a radio frequency at which the skin depth is many tens of meters, thereby overcoming the thermal diffusion times needed for conductive heating.[21][50][51] Its drawbacks include intensive electrical demand and the possibility that groundwater or char would absorb undue amounts of the energy.[21] Radio frequency processing in conjunction with critical fluids is being developed by Raytheon together with CF Technologies and tested by Schlumberger.[52][53]
Microwave heating technologies are based on the same principles as radio wave heating, although it is believed that radio wave heating is an improvement over microwave heating because its energy can penetrate farther into the oil shale formation.[54] The microwave heating process is being tested by Global Resource Corporation.[55] Electro-Petroleum proposes electrically enhanced oil recovery by the passage of direct current between cathodes in producing wells and anodes located either at the surface or at depth in other wells. The passage of the current through the oil shale formation results in resistive Joule heating.[11]
Economics
The dominant question for shale oil production is under what conditions shale oil is economically viable. The various attempts to develop oil shale deposits have succeeded only when the shale-oil production cost in a given region is lower than the price of petroleum or its other substitutes. According to a survey conducted by the RAND Corporation, the cost of producing a barrel of shale oil at a hypothetical surface retorting complex in the United States (comprising a mine, retorting plant, upgrading plant, supporting utilities, and spent shale reclamation), would range between US$70–95 ($440–600/m3), adjusted to 2005 values. Assuming a gradual increase in output after the start of commercial production, the analysis projects a gradual reduction in processing costs to $30–40 per barrel ($190–250/m3) after achieving the milestone of 1 billion barrels (160×106 m3).[9][42] Royal Dutch Shell has announced that its Shell ICP technology would realize a profit when crude oil prices are higher than $30 per barrel ($190/m3), while some technologies at full-scale production assert profitability at oil prices even lower than $20 per barrel ($130/m3).[12][56]
To increase the efficiency of oil shale retorting and by this the viability of the shale oil production, researchers have proposed and tested several co-pyrolysis processes, in which other materials such as biomass, peat, waste bitumen, or rubber and plastic wastes are retorted along with the oil shale.[57][58][59][60][61] Some modified technologies propose combining a fluidized bed retort with a circulated fluidized bed furnace for burning the by-products of pyrolysis (char and oil shale gas) and thereby improving oil yield, increasing throughput, and decreasing retorting time.[62]
A critical measure of the viability of oil shale as an energy source lies in the ratio of the energy produced by the shale to the energy used in its mining and processing, a ratio known as "Energy Returned on Energy Invested" (EROEI). A 1984 study estimated the EROEI of the various known oil shale deposits as varying between 0.7–13.3;[63] some companies and newer technologies assert an EROEI between 3 and 10. To increase the EROEI, several combined technologies were proposed. These include the usage of process waste heat, e.g. gasification or combustion of the residual carbon (char), and the usage of waste heat from other industrial processes, such as coal gasification and nuclear power generation.[11][64][65] The water needed in some extraction processes offers an additional economic consideration: this may pose a problem in areas with water scarcity.
Environmental considerations
Objections to its potential environmental impact have stalled governmental support for extraction of shale oil in some countries, e.g. Australia.[66] Shale oil extraction may involve a number of different environmental impacts that vary with process technologies. Depending on the geological conditions and mining techniques, mining impacts may include acid drainage induced by the sudden rapid exposure and subsequent oxidation of formerly buried materials, the introduction of metals into surface water and groundwater, increased erosion, sulfur gas emissions, and air pollution caused by the production of particulates during processing, transport, and support activities.[50][67] Surface mining for ex situ processing, as with in situ processing, requires extensive land use and ex situ thermal processing generates wastes that require disposal. Mining, processing, spent shale disposal, and waste treatment require land to be withdrawn from traditional uses and should therefore avoid areas of high population density.[9][68] Depending on the processing technology, the waste material may contain pollutants including sulfates, heavy metals, and polycyclic aromatic hydrocarbons, some of which are toxic and carcinogenic.[69][70] Experimental in situ conversion processes may reduce some of these impacts, but may instead cause other problems, such as groundwater pollution.
The production and usage of oil shale usually generates more greenhouse gas emissions, including carbon dioxide, than conventional fossil fuels.[68] Depending on the technology and the oil shale composition, shale oil extraction may create also sulfur dioxide, hydrogen sulfide, carbonyl sulfide, and nitrogen oxides emissions.[71] Developing carbon capture and storage technologies may reduce the processes' carbon footprint.[72]
Concerns have been prominently raised over the oil shale industry's use of water, particularly in arid regions where water consumption is a sensitive issue.[73] In some cases, oil shale mining requires the lowering of groundwater levels below the level of the oil shale strata, which may affect the surrounding arable land and forest.[9] Above-ground retorting typically consumes between one and five barrels of water per barrel of produced shale oil, depending on technology.[42][74] Water is usually used for spent shale cooling and oil shale ash disposal. In situ processing, according to one estimate, uses about one-tenth as much water.[75]
A 2008 programmatic environmental impact statement issued by the United States Bureau of Land Management stated that surface mining and retort operations produce 2 to 10 US gallons (7.6 to 38 l; 1.7 to 8.3 imp gal) of waste water per 1 short ton (0.91 t) of processed oil shale.[74]
See also
- Oil shale geology
- Oil shale reserves
- Oil shale in China
- Oil shale in Estonia
- Oil shale in Jordan
References
- ↑ 1.0 1.1 1.2 1.3 1.4 Louw, S.J.; Addison, J. (1985). Seaton, A.. ed (PDF). Studies of the Scottish oil shale industry. Vol.1 History of the industry, working conditions, and mineralogy of Scottish and Green River formation shales. Final report on US Department of Energy. Institute of Occupational Medicine. pp. 35; 38; 56–57. DE-ACO2 – 82ER60036. http://www.iom-world.org/pubs/IOM_TM8502.pdf. Retrieved 2009-06-05.
- ↑ 2.0 2.1 (PDF) Oil Shale. Colorado School of Mines. 2008. http://emfi.mines.edu/emfi2008/OilShale2008.pdf?CMSPAGE=outreach/cont_ed/emfi/emfi2008/OilShale2008.pdf. Retrieved 2008-12-24.
- ↑ Forbes, R.J. (1970). A Short History of the Art of Distillation from the Beginnings Up to the Death of Cellier Blumenthal. Brill Publishers. pp. 41–42. ISBN 9789004006171. http://books.google.com/books?id=u_tui-7XXF0C&pg=PA41. Retrieved 2009-06-02.
- ↑ Moody, Richard (2007-04-20) (PDF). Oil & Gas Shales, Definitions & Distribution In Time & Space. In The History of On-Shore Hydrocarbon Use in the UK. Geological Society of London. p. 1. http://www.geolsoc.org.uk/webdav/site/GSL/shared/pdfs/specialist%20and%20regional%20groups/hogg_weymouth.pdf. Retrieved 2007-07-28.
- ↑ Cane, R.F. (1976). "The origin and formation of oil shale". In Teh Fu Yen; Chilingar, George V.. Oil Shale. Amsterdam: Elsevier. p. 56. ISBN 9780444414083. http://books.google.com/books?id=qkU7OcVkwaIC&pg=PA56. Retrieved 2009-06-05.
- ↑ Runnels, Russell T.; Kulstad, Robert O.; McDuffee, Clinton; Schleicher, John A. (1952). "Oil Shale in Kansas". Kansas Geological Survey Bulletin (University of Kansas Publications) (96, part 3). http://www.kgs.ku.edu/Publications/Bulletins/96_3/index.html. Retrieved 2009-05-30.
- ↑ 7.0 7.1 7.2 Dyni, John R. (2007). "Oil Shale". In Clarke, A. W.; Trinnaman, J. A. (PDF). Survey of energy resources (21 ed.). World Energy Council. pp. 93–115. ISBN 0946121265. http://www.worldenergy.org/documents/ser2007_final_online_version_1.pdf. Retrieved 2007-11-13.
- ↑ 8.0 8.1 Prien, Charles H. (1976). "Survey of oil-shale research in last three decades". In Teh Fu Yen; Chilingar, George V.. Oil Shale. Amsterdam: Elsevier. pp. 237–243. ISBN 9780444414083. http://books.google.com/books?id=qkU7OcVkwaIC&pg=PA237. Retrieved 2009-06-05.
- ↑ 9.0 9.1 9.2 9.3 Francu, Juraj; Harvie, Barbra; Laenen, Ben; Siirde, Andres; Veiderma, Mihkel (May 2007) (PDF). A study on the EU oil shale industry viewed in the light of the Estonian experience. A report by EASAC to the Committee on Industry, Research and Energy of the European Parliament. European Academies Science Advisory Council. pp. 12–13; 18–19; 23–24; 28. http://www.easac.org/fileadmin/PDF_s/reports_statements/Study.pdf. Retrieved 2010-06-21.
- ↑ 10.00 10.01 10.02 10.03 10.04 10.05 10.06 10.07 10.08 10.09 10.10 10.11 (PDF) An Assessment of Oil Shale Technologies. DIANE Publishing. June 1980. pp. 108–110; 133; 138–139; 148–150. NTIS order #PB80-210115. ISBN 9781428924635. http://www.princeton.edu/~ota/disk3/1980/8004/8004.PDF. Retrieved 2007-11-03.
- ↑ 11.00 11.01 11.02 11.03 11.04 11.05 11.06 11.07 11.08 11.09 11.10 11.11 11.12 11.13 11.14 11.15 (PDF) Secure Fuels from Domestic Resources: The Continuing Evolution of America's Oil Shale and Tar Sands Industries. United States Department of Energy, Office of Naval Petroleum and Oil Shale Reserves. 2007. pp. 3; 8; 16–17; 22–29; 36–37; 40–43; 54–57. http://fossil.energy.gov/programs/reserves/npr/Secure_Fuels_from_Domestic_Resources_-_P.pdf. Retrieved 2007-07-11.
- ↑ 12.0 12.1 12.2 12.3 12.4 12.5 12.6 12.7 12.8 Johnson, Harry R.; Crawford, Peter M.; Bunger, James W. (2004) (PDF). Strategic significance of America's oil shale resource. Volume II: Oil shale resources, technology and economics. Office of Deputy Assistant Secretary for Petroleum Reserves; Office of Naval Petroleum and Oil Shale Reserves; United States Department of Energy. pp. 13–16; A2; B3–B5. http://www.fossil.energy.gov/programs/reserves/npr/publications/npr_strategic_significancev2.pdf. Retrieved 2007-06-23.
- ↑ Bureau of Land Management (2005-09-20). "Nominations for Oil Shale Research Leases Demonstrate Significant Interest in Advancing Energy Technology". Press release. http://www.blm.gov/wo/st/en/info/newsroom/2005/september/NR_050920.html. Retrieved 2007-07-10.
- ↑ Brendow, K. (2009). "Oil shale – a local asset under global constraint" (PDF). Oil Shale. A Scientific-Technical Journal (Estonian Academy Publishers) 26 (3): 357–372. doi:10.3176/oil.2009.3.02. ISSN 0208-189X. http://www.kirj.ee/public/oilshale_pdf/2009/issue_3/oil-2009-3-357-372.pdf. Retrieved 2009-09-25.
- ↑ 15.0 15.1 15.2 15.3 15.4 15.5 Qian Jialin; Wang Jianqiu (2006-11-07). "World oil shale retorting technologies" (PDF). International Oil Shale Conference. Amman, Jordan: Jordanian Natural Resources Authority. http://www.sdnp.jo/International_Oil_Conference/rtos-A118.pdf. Retrieved 2007-06-29.
- ↑ 16.0 16.1 Aarna, Indrek (2009). "Editor's page. The 3rd International Oil Shale Symposium in Tallinn" (PDF). Oil Shale. A Scientific-Technical Journal (Estonian Academy Publishers) 26 (3): 349–356. doi:10.3176/oil.2009.3.01. ISSN 0208-189X. http://www.kirj.ee/public/oilshale_pdf/2009/issue_3/oil-2009-3-349-356.pdf. Retrieved 2009-09-25.
- ↑ Luck, Taylor (2008-08-07). "Jordan set to tap oil shale potential". The Jordan Times (Jordan Press Foundation). http://www.jordantimes.com/index.php?news=9860. Retrieved 2008-10-25.
- ↑ "San Leon Energy Awarded Moroccan Oil Shale Exploration Project". OilVoice (OilVoice). 2009-06-01. http://www.oilvoice.com/n/San_Leon_Energy_Awarded_Moroccan_Oil_Shale_Exploration_Project/05a3d3f1.aspx. Retrieved 2009-06-03.
- ↑ 19.0 19.1 19.2 19.3 19.4 Koel, Mihkel (1999). "Estonian oil shale". Oil Shale. A Scientific-Technical Journal (Estonian Academy Publishers) (Extra). ISSN 0208-189X. http://www.kirj.ee/public/oilshale/Est-OS.htm. Retrieved 2007-07-21.
- ↑ 20.0 20.1 20.2 Luik, Hans (2009-06-08). "Alternative technologies for oil shale liquefaction and upgrading" (PDF). International Oil Shale Symposium. Tallinn, Estonia. http://www.oilshalesymposium.com/fileadmin/user_upload/documents/LUIK_2.pdf. Retrieved 2009-06-09.
- ↑ 21.00 21.01 21.02 21.03 21.04 21.05 21.06 21.07 21.08 21.09 21.10 21.11 21.12 21.13 Burnham, Alan K.; McConaghy, James R. (2006-10-16). "Comparison of the acceptability of various oil shale processes" (PDF). 26th Oil shale symposium. Golden, Colorado. pp. 2; 17. UCRL-CONF-226717. https://e-reports-ext.llnl.gov/pdf/341283.pdf. Retrieved 2007-05-27.
- ↑ 22.0 22.1 "Synthetic Fuels Summary. Report No. FE-2468-82" (PDF). The Engineering Societies Commission on Energy, Inc. (United States Department of Energy): 80; 83–84; 90. March 1981. http://www.fischer-tropsch.org/DOE/DOE_reports/10679_t10/10679_t10_sec05.pdf. Retrieved 2009-07-17.
- ↑ Gorlov, E.G. (October 2007). "Thermal Dissolution Of Solid Fossil Fuels" (PDF). Solid Fuel Chemistry (Allerton Press, Inc.) 41 (5): 290–298. doi:10.3103/S0361521907050047. ISSN 1934-8029. http://www.springerlink.com/content/n06l546490wt3544/fulltext.pdf?page=1. Retrieved 2009-06-09.
- ↑ Koel, Mihkel; Ljovin, S.; Hollis, K.; Rubin, J. (2001). "Using neoteric solvents in oil shale studies" (PDF). Pure and Applied Chemistry (Blackwell Science) 73 (1): 153–159. doi:10.1351/pac200173010153. ISSN 0033-4545. http://old.iupac.org/publications/pac/2001/pdf/7301x0153.pdf. Retrieved 2010-01-22.
- ↑ Baldwin, R. M.; Bennett, D. P.; Briley, R. A. (1984). "Reactivity of oil shale towards solvent hydrogenation" (PDF). American Chemical Society. Division of Petroleum Chemistry (American Chemical Society) 29 (1): 148–153. ISSN 0569-3799. http://www.osti.gov/energycitations/product.biblio.jsp?osti_id=6697587. Retrieved 2010-01-22.
- ↑ 26.0 26.1 26.2 Speight, James G. (2008). Synthetic Fuels Handbook: Properties, Process, and Performance. McGraw-Hill. pp. 182; 186. ISBN 9780071490238. http://books.google.com/books?id=E3pgqnGgHjIC&pg=PA182. Retrieved 2009-03-14.
- ↑ 27.0 27.1 27.2 27.3 Smith, M.W.; Shadle, L.J.; Hill, D. (2007) (PDF). Oil Shale Development from the Perspective of NETL's Unconventional Oil Resource Repository. United States Department of Energy. DOE/NETL-IR-2007-022. http://www.osti.gov/energycitations/servlets/purl/915351-0AdXs8/915351.pdf. Retrieved 2009-11-29.
- ↑ Fuels to drive our future. National Academies Press. 1990. p. 183. ISBN 9780309086455. http://books.nap.edu/openbook.php?record_id=1440&page=183. Retrieved 2008-05-04.
- ↑ 29.0 29.1 29.2 "Appendix A: Oil Shale Development Background and Technology Overview" (PDF). Proposed Oil Shale and Tar Sands Resource Management Plan Amendments to Address Land Use Allocations in Colorado, Utah, and Wyoming and Final Programmatic Environmental Impact Statement. Bureau of Land Management. September 2008. pp. 36; 54−55. doi:FES 08-32. http://ostseis.anl.gov/documents/fpeis/vol3/OSTS_FPEIS_vol3_App_A.pdf. Retrieved 2010-08-07.
- ↑ Soone, Jüri; Riisalu, Hella; Kekisheva, Ljudmilla; Doilov, Svjatoslav (2006-11-07). "Environmentally sustainable use of energy and chemical potential of oil shale" (PDF). International Oil Shale Conference. Amman, Jordan: Jordanian Natural Resources Authority. pp. 2–3. http://www.sdnp.jo/International_Oil_Conference/rtos-A104.pdf. Retrieved 2007-06-29.
- ↑ "Shale Oil". Commonwealth of Australia - Australian Mines Atlas. 2009. http://www.australianminesatlas.gov.au/aimr/commodity/shale_oil_09.jsp. Retrieved 2010-01-15.
- ↑ Coates, Ralph L.; Hatfield, Kent E.; Smoot, L. Douglas (2007-10-16). "A New Improved Process for Processing Oil Shale Ore into Motor Ready Fuel Products" (PDF). 27th Oil Shale Symposium. Golden, Colorado: Colorado School of Mines. http://www.ceri-mines.org/documents/27symposium/presentations/av07-1hatfield.pdf. Retrieved 2009-04-12.
- ↑ Coates, Ralph L.; Hatfield, Kent E.; Smoot, L. Douglas (2007-10-17). "A method of reducing CO2 emissions from oil shale retorting" (PDF). 27th Oil Shale Symposium. Golden, Colorado: Colorado School of Mines. http://www.ceri-mines.org/documents/27symposium/presentations/av15-4coates.pdf. Retrieved 2009-04-12.
- ↑ Biglarbigi, Khosrow; Mohan, Hitesh; Crawford, Peter; Carolus, Marshall (2008-12-04). "Economics, Barriers, and Risks of Oil Shale Development in the United States" (PDF). 28th United States Association for Energy Economics/International Association for Energy Economics North America Conference. New Orleans: The United States Association for Energy Economics. http://www.usaee.org/usaee2008/submissions/OnlineProceedings/7995-Biglarbigi%20-%20Oil%20Shale%20Economics.pdf. Retrieved 2009-09-27.
- ↑ 35.0 35.1 35.2 Crawford, Peter M.; Biglarbigi, Khosrow; Killen, James R.; Dammer, Anton R.; Knaus, Emily (2008-09-22). "Advances in World Oil-Shale Production Technologies". Society of Petroleum Engineers Annual Technical Conference and Exhibition. Denver, Colorado: Society of Petroleum Engineers.
- ↑ Laherrère, Jean H. (2005) (PDF). Review on oil shale data. Hubbert Peak. http://www.hubbertpeak.com/laherrere/OilShaleReview200509.pdf. Retrieved 2007-06-17.
- ↑ Rex, R.; Janka, J. C.; Knowlton, T. (1984). Cold Flow Model Testing of the Hytort Process Retort Design. 17th Oil Shale Symposium. Golden, Colorado: Colorado School of Mines Press. pp. 17–36.
- ↑ Weil, S. A.; Feldkirchner, H. L.; Punwani, D. V.; Janka, J. C. (1979-01-01). IGT HYTORT Process for hydrogen retorting of Devonian oil shales. Chicago: Gas Technology Institute. CONF-790571-3.
- ↑ Kök, M. V.; Guner, G.; Suat Bağci, A. (2008). "Application of EOR techniques for oil shale fields (in-situ combustion approach)" (PDF). Oil Shale. A Scientific-Technical Journal (Estonian Academy Publishers) 25 (2): 217–225. doi:10.3176/oil.2008.2.04. http://www.kirj.ee/public/oilshale_pdf/2008/issue_2/oil-2008-2-217-225.pdf. Retrieved 2008-06-07.
- ↑ 40.0 40.1 Savage, Marshall T. (2006-10-17). "Geothermic fuel cells" (PDF). 26th Oil Shale Symposium. Golden, Colorado: Colorado School of Mines/. http://www.ceri-mines.org/documents/R05d-MarshallSavage.pdf. Retrieved 2009-09-25.
- ↑ 41.0 41.1 Lee, Sunggyu; Speight, James G.; Loyalka, Sudarshan K. (2007). Handbook of Alternative Fuel Technologies. CRC Press. p. 290. ISBN 9780824740696. http://books.google.com/books?id=hyNbv60Px8oC&pg=PA290. Retrieved 2009-03-14.
- ↑ 42.0 42.1 42.2 42.3 Bartis, James T.; LaTourrette, Tom; Dixon, Lloyd; Peterson, D.J.; Cecchine, Gary (2005) (PDF). Oil Shale Development in the United States. Prospects and Policy Issues. Prepared for the National Energy Technology Laboratory of the United States Department of Energy. The RAND Corporation. pp. x; 15–18; 50. ISBN 978-0-8330-3848-7. http://www.rand.org/pubs/monographs/2005/RAND_MG414.pdf. Retrieved 2007-06-29.
- ↑ Birger, Jon (2007-11-01). "Oil shale may finally have its moment". Fortune. http://money.cnn.com/2007/10/30/magazines/fortune/Oil_from_stone.fortune/. Retrieved 2007-11-17.
- ↑ Reiss, Spencer (2005-12-13). "Tapping the Rock Field". WIRED magazine. http://www.wired.com/wired/archive/13.12/oilshale.html. Retrieved 2009-03-14.
- ↑ (PDF) Plan of Operation for Oil Shale Research, Development and Demonstration (R,D/D) Tract. E.G.L. Resources, Inc.. 2006-02-15. http://www.blm.gov/pgdata/etc/medialib/blm/co/field_offices/white_river_field/oil_shale.Par.62160.File.dat/PlanofOperation.pdf. Retrieved 2008-05-01.
- ↑ (PDF) Oil Shale Research, Development & Demonstration Project. Plan of Operation. Chevron USA, Inc.. 2006-02-15. http://www.blm.gov/pgdata/etc/medialib/blm/co/field_offices/white_river_field/oil_shale.Par.37256.File.dat/OILSHALEPLANOFOPERATIONS.pdf. Retrieved 2008-05-01.
- ↑ Doyle, Dave (March 2008). "Single well, single gas phase technique is key to unique method of extracting oil vapors from oil shale". World Oil Magazine (Gulf Publishing Company). (subscription required). http://www.worldoil.com/March-2008-Single-well-single-gas-phase-technique-is-key-to-unique-method-of-extracting-oil-vapors-from-oil-shale.html. Retrieved 2009-09-27.
- ↑ Plunkett, Jack W. (2008). Plunkett's Energy Industry Almanac 2009: The Only Comprehensive Guide to the Energy & Utilities Industry. Plunkett Research, Ltd.. p. 71. ISBN 9781593921286. http://books.google.com/books?id=Ut3zgub_PRwC&pg=PT71. Retrieved 2009-03-14.
- ↑ 49.0 49.1 Symington, William A.; Olgaard, David L.; Otten, Glenn A.; Phillips, Tom C.; Thomas, Michele M.; Yeakel, Jesse D. (2008-04-20). "ExxonMobil's Electrofrac Process for In Situ Oil Shale Conversion" (PDF). AAAPG Annual Convention. San Antonio: American Association of Petroleum Geologists. http://www.nevtahoilsands.com/pdf/Oil-Shale-and-Tar-Sands-Company-Profiles.pdf. Retrieved 2009-04-12.
- ↑ 50.0 50.1 Burnham, Alan K. (2003-08-20) (PDF). Slow Radio-Frequency Processing of Large Oil Shale Volumes to Produce Petroleum-like Shale Oil. Lawrence Livermore National Laboratory. UCRL-ID-155045. https://e-reports-ext.llnl.gov/pdf/243505.pdf. Retrieved 2007-06-28.
- ↑ Carlson, R. D.; Blase, E. F.; McLendon, T. R. (1981-04-22). "Development of the IIT Research Institute RF heating process for in situ oil shale/tar sand fuel extraction–an overview". Oil Shale Symposium Proceedings. 14th Oil Shale Symposium (Golden, Colorado: Colorado School of Mines): 138–145. CONF-810456.
- ↑ (PDF) Radio Frequency/Critical Fluid Oil Extraction Technology. Raytheon. http://www.raytheon.com/businesses/rids/products/rtnwcm/groups/public/documents/content/rtn_bus_ids_prod_rfcf_pdf.pdf. Retrieved 2008-08-20.
- ↑ Moon, Ted (2008-02-01). "Oil-shale extraction technology has a new owner". The Journal of Petroleum Technology (Society of Petroleum Engineers). http://www.spe.org/jpt/2008/02/oil-shale-extraction-technology-has-a-new-owner/. Retrieved 2008-08-20.
- ↑ Daniel, David Edwin; Lowe, Donald F.; Oubre, Carroll L.; Ward, Calvin Herbert (1999). Soil vapor extraction using radio frequency heating: resource manual and technology demonstration. CRC Press. p. 1. ISBN 9781566704649. http://books.google.com/books?hl=en&lr=&id=vd8EIXX-OOQC&oi=fnd&pg=PA1. Retrieved 2009-09-26.
- ↑ Global Resource Corp. (2007-03-09). "Global Resource Reports Progress on Oil Shale Conversion Process". Press release. http://www.downstreamtoday.com/news/article.aspx?a_id=1943. Retrieved 2008-05-31.
- ↑ Schmidt, S. J. (2003). "New directions for shale oil:path to a secure new oil supply well into this century: on the example of Australia" (PDF). Oil Shale. A Scientific-Technical Journal (Estonian Academy Publishers) 20 (3): 333–346. ISSN 0208-189X. http://www.kirj.ee/public/oilshale/7_schmidt_2003_3s.pdf. Retrieved 2007-06-02.
- ↑ Tiikma, Laine; Johannes, Ille; Pryadka, Natalja (2002). "Co-pyrolysis of waste plastics with oil shale". Proceedings. Symposium on Oil Shale 2002, Tallinn, Estonia: 76.
- ↑ Tiikma, Laine; Johannes, Ille; Luik, Hans (March 2006). "Fixation of chlorine evolved in pyrolysis of PVC waste by Estonian oil shales". Journal of Analytical and Applied Pyrolysis 75 (2): 205–210. doi:10.1016/j.jaap.2005.06.001.
- ↑ Veski, R.; Palu, V.; Kruusement, K. (2006). "Co-liquefaction of kukersite oil shale and pine wood in supercritical water" (PDF). Oil Shale. A Scientific-Technical Journal (Estonian Academy Publishers) 23 (3): 236–248. ISSN 0208-189X. http://www.kirj.ee/public/oilshale/oil-2006-3-4.pdf. Retrieved 2007-06-16.
- ↑ Aboulkas, A.; El Harfi, K.; El Bouadili, A.; Benchanaa, M.; Mokhlisse, A.; Outzourit, A. (2007). "Kinetics of co-pyrolysis of Tarfaya (Morocco) oil shale with high-density polyethylene" (PDF). Oil Shale. A Scientific-Technical Journal (Estonian Academy Publishers) 24 (1): 15–33. ISSN 0208-189X. http://www.kirj.ee/public/oilshale/oil-2006-3-4.pdf. Retrieved 2007-06-16.
- ↑ Ozdemir, M.; A. Akar, A. Aydoğan, E. Kalafatoglu; E. Ekinci (2006-11-07). "Copyrolysis of Goynuk oil shale and thermoplastics" (PDF). International Oil Shale Conference. Amman, Jordan: Jordanian Natural Resources Authority. http://www.sdnp.jo/International_Oil_Conference/rtos-A114.pdf. Retrieved 2007-06-29.
- ↑ Siirde, Andres; Martins, Ants (2009-06-07). "Oil shale fluidized bed retorting technology with CFB furnace for buring the by-products" (PDF). International Oil Shale Symphosium. Tallinn, Estonia: Tallinn University of Technology. http://www.oilshalesymposium.com/fileadmin/user_upload/documents/SIIRDE.pdf. Retrieved 2009-05-22.
- ↑ Cleveland, Cutler J.; Costanza, Robert; Hall, Charles A. S.; Kaufmann, Robert (1984-08-31). "Energy and the U.S. Economy: A Biophysical Perspective". Science (American Association for the Advancement of Science) 225 (4665): 890–897. doi:10.1126/science.225.4665.890. PMID 17779848.
- ↑ Parkinson, Gerald (2007). "Oil Shale: The U.S. Takes Another Look at a Huge Domestic Resource". Chemical Engineering Progress (American Institute of Chemical Engineers) 102 (7). http://findarticles.com/p/articles/mi_qa5350/is_200607/ai_n21394714. Retrieved 2008-08-21.
- ↑ Clark, Judy (2008-08-11). "Nuclear heat advances oil shale refining in situ". Oil & Gas Journal (PennWell Corporation) 106 (30): pp. 22–24. (subscription required). http://www.ogj.com/index/article-display/336580/s-articles/s-oil-gas-journal/s-volume-106/s-issue-30/s-general-interest/s-nuclear-heat-advances-oil-shale-refining-in-situ.html. Retrieved 2009-05-23.
- ↑ "Bligh bans Whitsundays shale oil mining". ABC News (The Australian Broadcasting Corporation). 2008-08-24. http://www.abc.net.au/news/stories/2008/08/24/2344733.htm?section=justin. Retrieved 2009-09-19.
- ↑ "Environmental Impacts from Mining" (PDF). The Abandoned Mine Site Characterization and Cleanup Handbook. United States Environmental Protection Agency. August 2000. pp. 3-1–3-11. http://www.epa.gov/superfund/policy/remedy/pdfs/amscch.pdf. Retrieved 2010-06-21.
- ↑ 68.0 68.1 (PDF) Driving It Home. Choosing the Right Path for Fueling North America's Transportation Future. Natural Resources Defense Council. June 2007. http://www.nrdc.org/energy/drivingithome/drivingithome.pdf. Retrieved 2008-04-19.
- ↑ Mölder, Leevi (2004). "Estonian Oil Shale Retorting Industry at a Crossroads" (PDF). Oil Shale. A Scientific-Technical Journal (Estonian Academy Publishers) 21 (2): 97–98. ISSN 0208-189X. http://www.kirj.ee/public/oilshale/1_ed_page_2004_2.pdf. Retrieved 2007-06-23.
- ↑ Tuvikene, Arvo; Huuskonen, Sirpa; Koponen, Kari; Ritola, Ossi; Mauer, Ülle; Lindström-Seppä, Pirjo (1999). "Oil Shale Processing as a Source of Aquatic Pollution: Monitoring of the Biologic Effects in Caged and Feral Freshwater Fish" (PDF). Environmental Health Perspectives (National Institute of Environmental Health Sciences) 107 (9): 745–752. doi:10.2307/3434660. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC1566439/pdf/envhper00514-0093.pdf. Retrieved 2007-06-16.
- ↑ Environmental consequences of, and control processes for, energy technologies. William Andrew Inc. 1990. p. 104. ISBN 9780815512318. http://books.google.com/books?id=qgFtunVE5T8C&pg=PA104&lpg=PA104. Retrieved 2008-08-19.
- ↑ Bartis, Jim (2006-10-26). "Unconventional Liquid Fuels Overview" (PDF). World Oil Conference. Boston: Association for the Study of Peak Oil & Gas - USA. http://www.aspo-usa.com/fall2006/presentations/pdf/Bartis_J_Boston_2006.pdf. Retrieved 2007-06-28.
- ↑ Speckman, Stephen (2008-03-22). "Oil-shale 'rush' is sparking concern". Deseret News (Deseret News Publishing Co.). ISSN 0745-4724. http://www.deseretnews.com/article/695263708/Oil-shale-rush-is-sparking-concern.html. Retrieved 2008-08-24.
- ↑ 74.0 74.1 "Chapter 4. Effects of Oil Shale Technologies" (PDF). Proposed Oil Shale and Tar Sands Resource Management Plan Amendments to Address Land Use Allocations in Colorado, Utah, and Wyoming and Final Programmatic Environmental Impact Statement. Bureau of Land Management. September 2008. pp. 4‑3. doi:FES 08-32. http://ostseis.anl.gov/documents/fpeis/vol1/OSTS_FPEIS_Vol1_Ch4.pdf. Retrieved 2010-08-07.
- ↑ Fischer, Perry A. (August 2005). "Hopes for shale oil are revived". World Oil Magazine (Gulf Publishing Company). Archived from the original on 2008-06-17. http://web.archive.org/web/20080617004331/http://www.worldoil.com/magazine/MAGAZINE_DETAIL.asp?ART_ID=2658&MONTH_YEAR=Aug-2005. Retrieved 2010-06-21.
External links
- Oil Shale. A Scientific-Technical Journal (ISSN 0208-189X)
- Oil Shale and Tar Sands Programmatic Environmental Impact Statement (EIS) Information Center. Concerning potential leases of Federal oil sands lands in Utah and oil shale lands in Utah, Wyoming, and Colorado.
- "Shale Oil Now" Campaign. Links and articles on America's shale oil compiled by Jon Moseley
- The United States National Oil Shale Association (NOSA)
|
||||||||||||||||||||||||||||||||||||||